By: Pierre R. Delaere, MD, PhD, and Dirk Van Raemdonck, MD, PhD
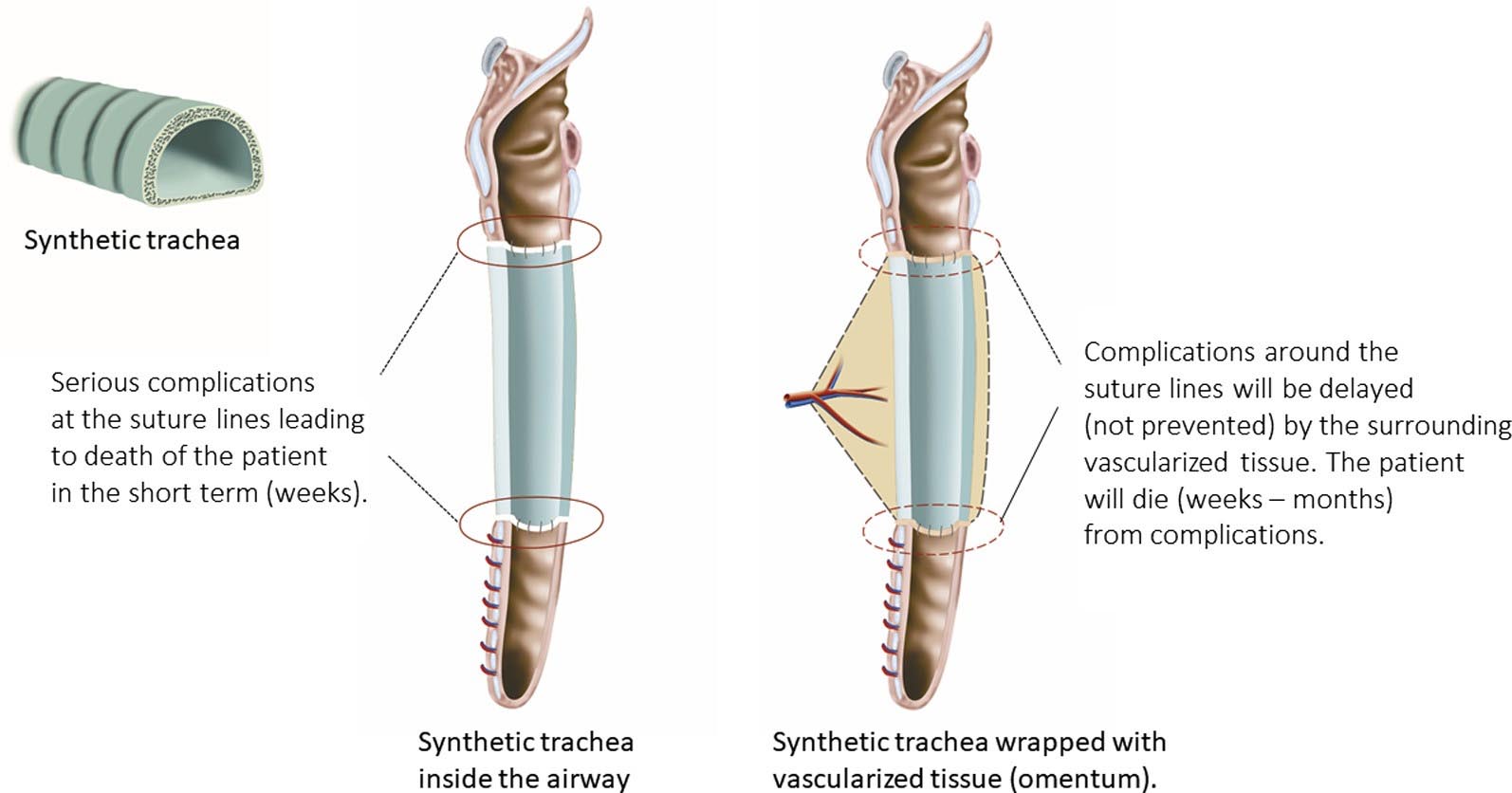
Figure 2 (top image): Left, nude synthetic trachea: suture lines will never heal, leading to anastomotic breakdown (red circles) with serious complications in the short term. Right, synthetic trachea wrapped with vascularized tissue (eg, omentum): suture lines will not heal. Anastomotic breakdown (red circles) will be de- layed by surrounding vascularized tissue. Serious complications will occur in the long term.
CENTRAL MESSAGE
Tracheal replacement remains a surgical challenge. Synthetic tracheas and nonvascularized donor tracheas are destined to fail and will never transform into a living functional organ.
FIGURE 1. Requirements for a regenerated trachea: (1) Mucosal lining; (2) support; (3) vascular supply.
Tracheal replacement to restore functional integrity of a long segmental defect or a complex airway stenosis remains a surgical challenge.1 A variety of surgical techniques have been reported clinically, including transplantation of autologous2 or heterologous3 tissue flaps, direct4,5 or indirect6 cadaveric tracheal allografting, and interposition of a ‘‘regenerated’’ trachea.7
In this issue of the Journal, Fux and colleagues8 from the Karolinska Institute in Stockholm publish a very detailed and well-illustrated long-term follow-up of 3 patients who received a ‘‘bioengineered tracheal allograft seeded with bone marrow cells’’ resulting in numerous complications and finally fatal outcome. These authors from Karolinska institute should be congratulated for their courage. However, the patients involved shouldn’t have undergone this procedure. The devastating outcome after the implantation of a synthetic trachea was fully predictable. In fact, the ‘‘bioengineered tracheal allograft seeded with bone marrow cells’’ was nothing more than a plastic tube.8
In 2008, a high-profile paper was published on the first successful regeneration of the trachea.8 In this case report,
2 miracles had occurred: (1) The cells of a donor trachea had been replaced by recipient cells after applying stem cells of the recipient to an enzymatically treated cadaver donor trachea; and (2) The ‘‘regenerated’’ trachea could be used for airway repair without restoration of its blood supply.
The paper attracted much media attention as the first organ that could be regenerated; it was foreseen that other hollow-organ regenerations would quickly follow.9 Indeed, the engineered trachea was seen to be the first step toward other forms of organ regeneration. Classic organ transplantations with their typical side effects due to antirejection medication could then be replaced by growing organs from the body’s own cells.
However, the problem with the tracheal regeneration concept, portrayed as successful, is that it is theoretically impossible. All requirements necessary to consider tracheal regeneration were lacking (Figure 1). More misleading high-profile papers were subsequently published in Lancet as the first ‘‘regenerated’’ synthetic trachea10 and the first ‘‘regenerated’’ cadaver trachea in a child.11 Since 2008, about 20 patients received a ‘‘regenerated’’ trachea in different centers around the world. As could be expected, severe complications arose, with fatal outcomes in the ma- jority of the patients.12 The tracheal replacements were initially published as successful in some patients because of the use of a stent and the omental wrapping, which de- layed the inevitable complications (Figure 2).
From the very beginning, we have tried to inform the medical world about this blatant example of scientific deception.13,14 From 2014 onwards, 4 whistleblowers of the Karolinska Institute, and the authors of this current pa- per in the Journal, joined us in these actions. We had to wait for a reaction from the involved institutions until January
2016, when the scandal was exposed to a wider audience after a 3-hour documentary that was aired on Swedish national television and on BBC. The overwhelming documentary resulted from top-shelf investigative journal- ism. Quickly after its broadcasting, board members of the Karolinska Institute, including the secretary-general of the Nobel Committee, had to resign and the paper on the regen- erated synthetic trachea was retracted.15,16
This misleading story on tissue regeneration hasn’t come to an end yet. Until now, other papers on the regenerated cadaver trachea still stay afloat. Synthetic tracheas and non- vascularized donor tracheas are destined to fail. They were wrongfully used for tracheal replacement and presented as breakthroughs in clinical tracheal tissue engineering.
References
- Grillo HC. Tracheal replacement: a critical review. Ann Thorac Surg. 2002;73: 1995-2004.
- Mercier O, Kolb F, Dartevelle PG. Autologous tracheal replacement: surgical technique and outcome. Thorac Surg Clin. 2018;28:347-55.
- Wurtz A, Porte H, Conti M, Desbordes J, Copin MC, Azorin J, et al. Tracheal replacement with aortic allografts. N Engl J Med. 2006;355:1938-40.
- Rose KG, Sesterhenn K, Wustrow F. Tracheal allotransplantation in man. Lancet. 1979;1:433.
- Levashov YN, Yablonsky PK, Cherny SM, Orlov SV, Shafirovsky BB, Kuznetzov IM. One stage allotransplantation of thoracic segment of the trachea in a patient with idiopathic fibrosing mediastinitis and marked tracheal stenosis. Eur J Cardiothorac Surg. 1993;7:383-6.
- Delaere P, Vranckx J, Verleden G, De Leyn P, Van Raemdonck D. Leuven Tracheal Transplant Group. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138-45.
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023-30.
- Fux T, O€sterholm C, Themudo R, Simonson O, Grinnemo K-H, Corbascio M. Synthetic tracheal grafts seeded with bone marrow cells fail to generate func- tional tracheae: first long-term follow-up study. J Thorac Cardiovasc Surg. October 31, 2019 [Epub ahead of print].
- Fountain H. A first: organs tailor-made with body’s own cells. The New York Times. September 15, 2012:A1. Available at: https://www.nytimes.com/2012/ 09/16/health/research/scientists-make-progress-in-tailor-made-organs.html. Ac- cessed October 27, 2019.
- Jungebluth P, Alici E, Baiguera S, Blomberg P, Boz’oky B, Crowley C, et al. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocom- posite: a proof-of-concept study. Lancet. 2011;378:1997-2004.
- Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, et al. Stem-cell-based tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994-1000.
- The For Better Science blog, ‘‘Macchiarini’s trachea transplant patients: the full list.’’ Available at: https://forbetterscience.com/2017/06/16/macchiarinis-trachea- transplant-patients-the-full-list/. Accessed October 27, 2019.
- Delaere PR, Van Raemdonck D. The trachea: the first tissue-engineered organ? J Thorac Cardiovasc Surg. 2014;147:1128-32.
- 90:928-9.Delaere P. Stem-cell ‘‘hype’’ in tracheal transplantation? Transplantation. 2010;
- The Lancet Editors. Retraction—Tracheobronchial transplantation with a stem-cell–seeded bioartificial nanocomposite: a proof-of-concept study. Lancet. 2018;392:11.
- Horton R. Offline: Paolo Macchiarini—science in conflict. Lancet. 2016;20:387.
From the Departments of a Oto-Rhino-Laryngology and b Thoracic Surgery, University Hospitals Leuven, Leuven, Belgium.
Disclosures: Authors have nothing to disclose with regard to commercial support. Received for publication Oct 27, 2019; revisions received Oct 27, 2019; accepted for publication Oct 28, 2019.
Address for reprints: Dirk Van Raemdonck, MD, PhD, Department of Thoracic Sur- gery, University Hospital Gasthuisberg, Herestraat 49, B-3000 Leuven, Belgium (E-mail: dirk.vanraemdonck@uzleuven.be).
J Thorac Cardiovasc Surg 2019;-:1-3 0022-5223/$36.00
Copyright © 2019 by The American Association for Thoracic Surgery https://doi.org/10.1016/j.jtcvs.2019.10.116